The first and only AI program cleared by the FDA that selectively amplifies brain lesion MR image contrast.
*AiMIFY performance has been proven with multiple gadolinium-based contrast agents and additional evidences will be added as per the AiMIFY predetermined change control plan
AiMIFY AI Software:
The MRI Contrast Amplifier for Brain Imagery
Imaging
Efficiency
Up to Double the Visibility,¹ with FDA-Approved Gd Dose†
AiMIFY software leverages deep-learning algorithms to predict and display amplified contrast enhancement between pre- and post-contrast image acquisition.¹
Average 100% increase in CEP‡
†AiMIFY-enhanced images are not fully equivalent to post-contrast images acquired with a higher than recommended actual dosage of contrast.
‡CEP = Contrast Enhancement Percentage.
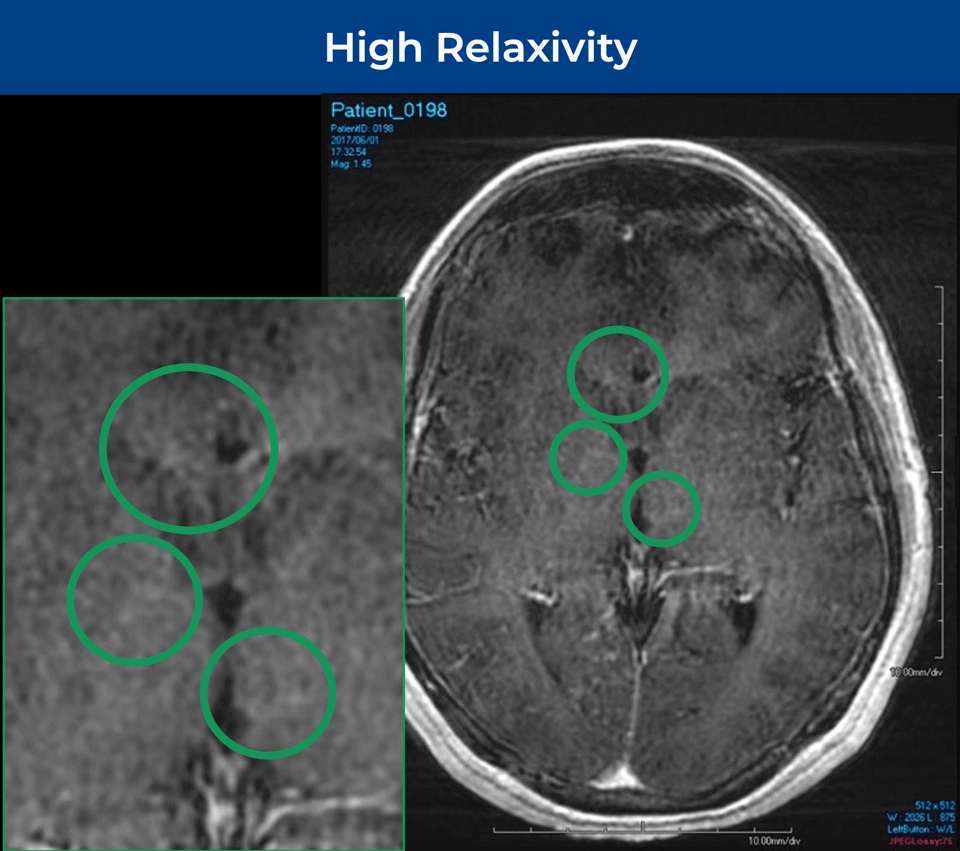
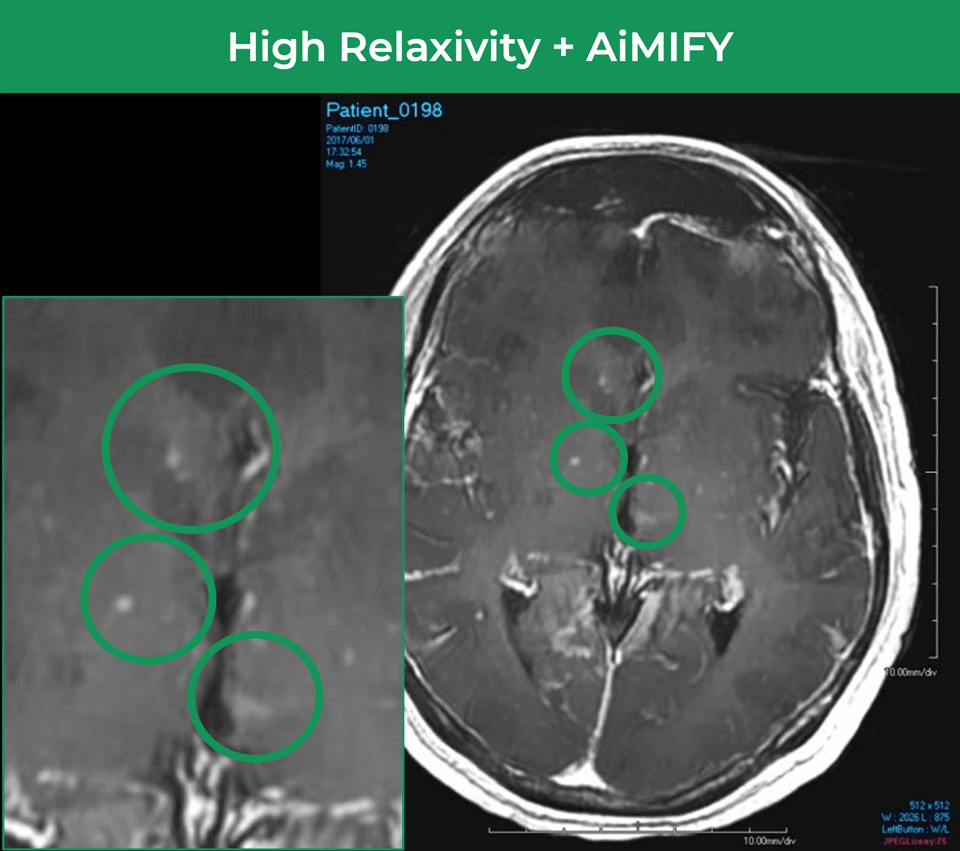
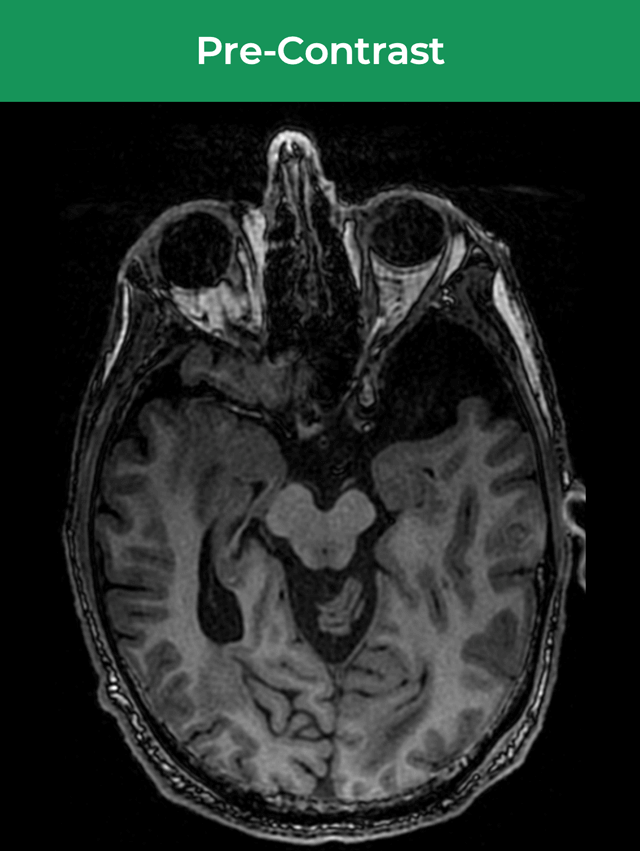
25-year-old female; GE Signa HDxt 1.5T scanner; concern for autoimmune encephalitis, cognitive decline + L hemiparesis.Å
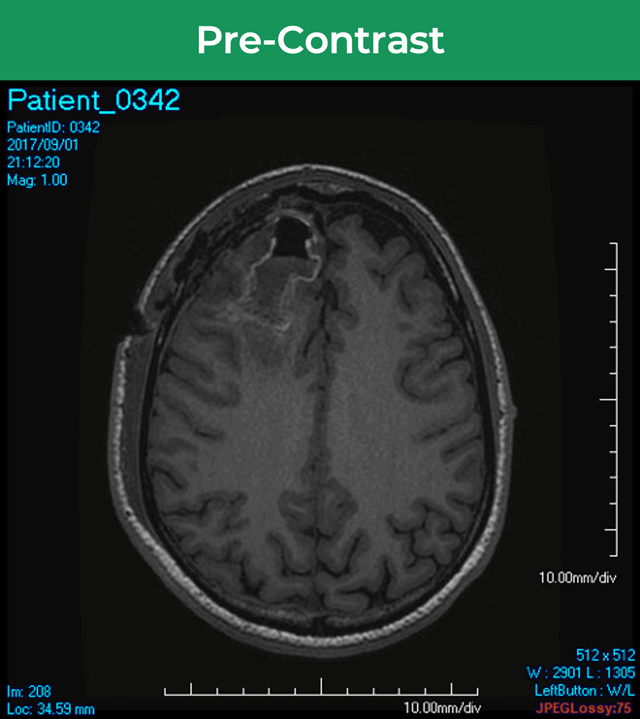
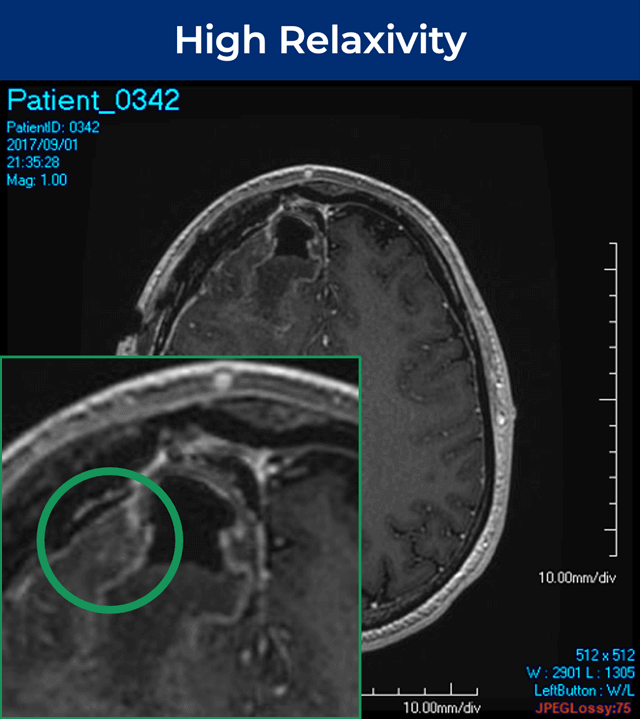
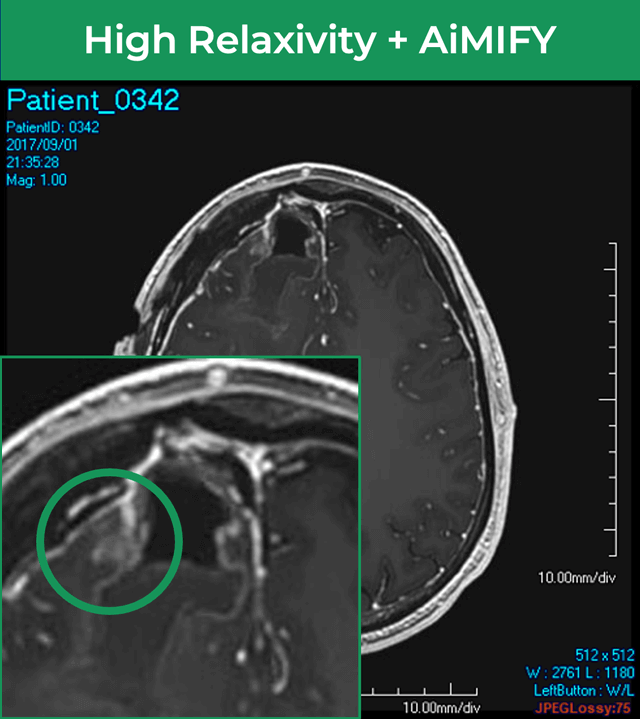
36-year-old male; GE Signa HDxt 1.5T scanner; glioma s/p resection.¹
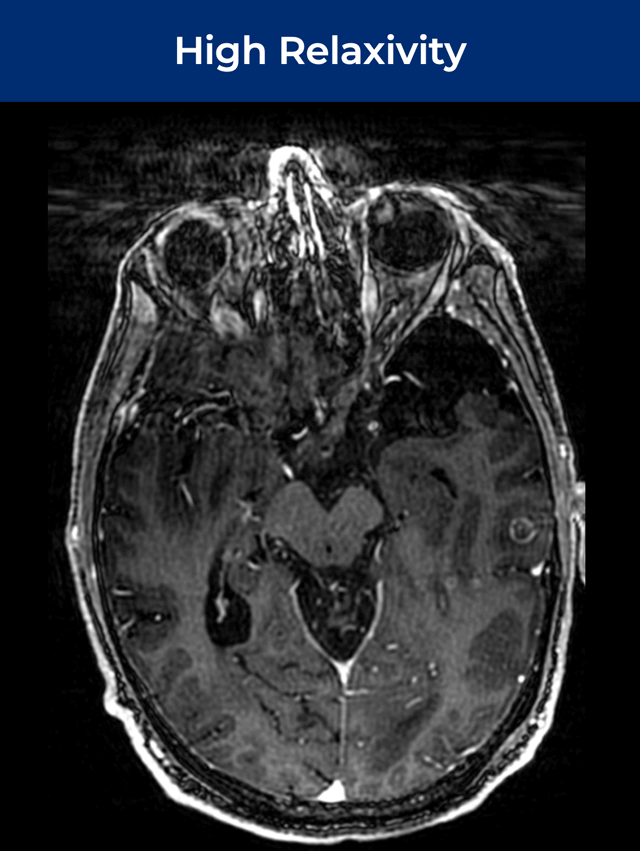
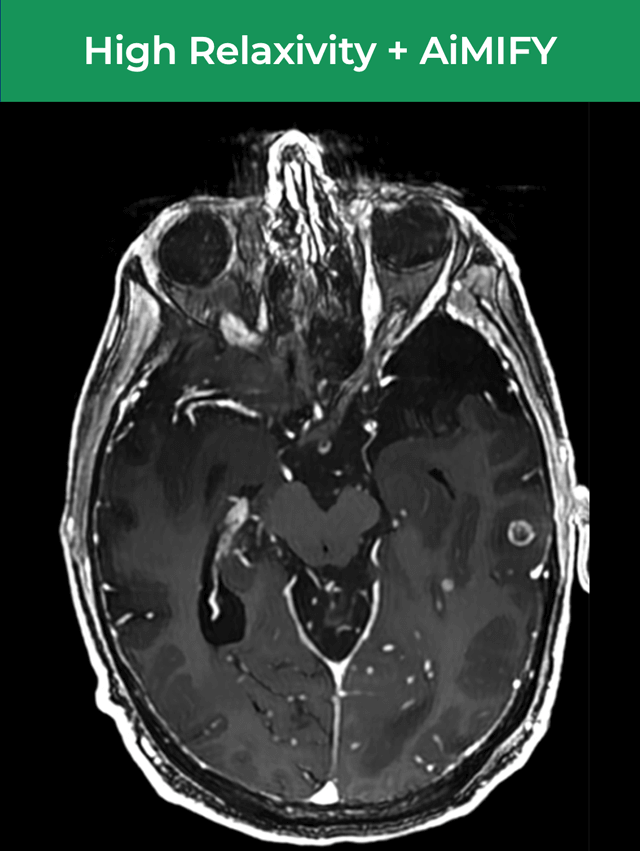
61-year-old male; GE Discovery MR750w 3T scanner; metastases.¹
Significantly Improves Brain Lesion Visibility¹—At FDA‑Approved Gd Dose†
- Identifies brain regions that take up Gd
- Selectively amplifies brain lesion visualization
- Up to double the visibility¹ of FDA-approved Gd doses†
†AiMIFY-enhanced images are not fully equivalent to post-contrast images acquired with a higher than recommended actual dosage of contrast.
hidden - keeps all closed on load
Seamless Integration into the Clinical Workflow
- No user interface
- Standard SubtleEDGE platform
- Cloud-based or on-premise
- Processing done after image acquisition
Can Support Neuroradiologists’ Diagnostic Confidence
- In challenging clinical cases
- In improving visualization of small lesions
- Improving quantitative image-quality metrics of contrast–enhanced brain imagesÅ
- Boosts contrast-to-noise ratio
- Amplifies lesion-to-brain ratio
- Improves contrast enhancement (CEP)
- Improves border delineation
- Increases internal morphology visualization
Helps You Achieve Higher Contrast Enhancement
Even after imaging is completed¹
- Can be used retrospectively, which can help reduce brain re-scans
- Offers a potentially safer alternative to increasing contrast beyond FDA-approved dosing
- Appropriate for brain imaging in patients aged 7 to 86, from adolescents through adults
Performance validated with:¹
- Small (<1 cc) and large (>1 cc) brain lesions
- Various MR sequences and acquisition orientations
- Leading magnet brands
FDA Class II Software as a Medical Device¹
- Does not require user input
- Lets you maintain your workflow: processing completed after image acquisition
- Compliant with DICOM Standard, ensures interoperability within PACS workflow
- Compliant with FDA cybersecurity guidelines and regulations
Amplify the Benefits of High
Relaxivity in Brain MRI
The MRI Contrast Amplifier for Brain Imagery
A partnership of the innovative imaging experts
• Bracco, your trusted imaging solutions partner
• Subtle Medical, a leader in AI imaging software
Please refer to the AiMIFY™ User Manual for full safety and use information.
For a demonstration, please contact your Bracco Representative.
Amplify the Benefits of High
Relaxivity in Brain MRI
The MRI Contrast Amplifier for Brain Imagery
A partnership of the innovative imaging experts
• Bracco, your trusted imaging solutions partner
• Subtle Medical, a leader in AI imaging software
Please refer to the AiMIFY™ User Manual for full safety and use information.
For a demonstration, please contact your Bracco Representative.
IMPORTANT SAFETY INFORMATION | AiMIFY™ Software
AiMIFY-enhanced images should not be used alone to assist patient diagnosis. The standard post-contrast image must always be reviewed first before using AiMIFY-enhanced images for patient diagnosis.
Please be aware that AiMIFY is not intended for artifact reduction, such as metal artifact and motion artifact. Enhanced images may have similar artifacts as input images.
Indications for Use
AiMIFY is an image processing software that can be used for image enhancement in MRI images. It can be used to increase contrast-to-noise ratio (CNR), contrast enhancement (CEP), and lesion-to-brain ratio (LBR) of enhancing tissue in brain MRI images acquired with a gadolinium-based contrast agent. It is intended to enhance MRI images acquired using standard approved dosage per the contrast agent’s instructions for use.
Cautions
AiMIFY-enhanced images should not be used alone to assist patient diagnosis. The standard post-contrast image must always be reviewed first before using AiMIFY-enhanced images for patient diagnosis.
Please be aware that AiMIFY is not intended for artifact reduction, such as metal artifact and motion artifact. Enhanced images may have similar artifacts as input images.
AiMIFY may enhance intensity differences between pre-and post-contrast images that are caused by 1) registration errors between the sequences or 2) image artifacts in either sequence.
AiMIFY may enhance vessel conspicuity more than standard acquired post-contrast images. In cases where vessel conspicuity interferes with or delays diagnosis, the standard post-contrast image should be used for adjudication.
AiMIFY-enhanced images may contain false lesions due to enhancement of image quality issues in either input sequences, due to being introduced by the AiMIFY algorithm, or both. The standard post-contrast image should be used to rule out false lesions.
Please be aware that AiMIFY has only been trained and tested on head MRI for contrast enhancement. Using the AiMIFY software in other anatomies or use cases not defined in this user manual could result in unknown image enhancement performance.
The internal morphology of tissue in input sequences have been tested to be moderately preserved by AiMIFY processing both for lesion tissue and parenchyma tissue. Internal morphology may appear to be missing due to window leveling of the image, and can be obtained by adjusting the window levels.
Please be aware that AiMIFY is intended for use only with standard of care quality images. Standard approved dose contrast images should be acquired per the contrast agent’s instructions for use. The standard of care should not be adjusted in preparation for using AiMIFY.
Please be aware that AiMIFY should not be used for acquiring stat scans or emergent scans, as image processing time may delay diagnosis.
Standard approved dose contrast images should be acquired per the contrast agent’s instructions for use. AiMIFY-enhanced images are not fully equivalent to post-contrast images acquired with a higher than recommended actual dosage of contrast. However, the high-dose-like appearance of AiMIFY-enhanced images offers a potentially safer alternative to actually acquiring post-contrast images beyond standard dosing.
Contraindications
AiMIFY is an image processing software that inherently doesn’t cause harm to patients of any subpopulation. However, gadolinium-based contrast agents are associated with contraindications, precautions, and warnings for certain subpopulations. Strictly follow the gadolinium-based contrast agent’s instructions for use, including but not limited to contraindicated patient populations, prior to processing with AiMIFY.
Limitations
AiMIFY has been trained and tested using a diverse DICOM image dataset collected from different MR imaging applications at 0.3T, 1.5T, and 3.0T, including:
- T1 weighted imaging for pre and post-contrast brain scans obtained with gadolinium-based contrast agents in 2D sequences, such as fluid-attenuated inversion recovery (FLAIR) and fast spin echo (FSE).
- T1 weighted imaging for pre and post-contrast brain scans obtained with gadolinium-based contrast agents in 3D sequences, such as BRAin VOlume (BRAVO) inversion-recovery-prep fast split echo (SPGR) and Magnetization Prepared Rapid Gradient Echo (MPRAGE).
- Acquisition in axial, coronal, and sagittal orientations.
- Images acquired with GE Medical Systems, Philips Medical Systems, Siemens Healthineers, and Hitachi scanners (see separate Scanner List for models).
- Images (for performance testing) spanning a variety of pathologies including patients with Cerebritis, Glioma, Inflammation, Lymphoma, Meningioma, Metastatic lesions, Multiple sclerosis, Neuritis, other tumor-related lesions (e.g., resection/radiation necrosis / suspected cyst / etc.), and other abnormalities.
- Patients aged 7 to 86 years old with an even distribution of females and males in the test dataset.
- Lesions in the dataset were small (<1 cc) and large (>1 cc).
Any imaging sequences/applications outside the above list may result in prolonged processing time and/or unreasonable results.
AiMIFY is manufactured for Bracco Diagnostics Inc. by Subtle Medical Inc. – Menlo Park, CA, USA 94025.
AiMIFY is a trademark of Bracco Imaging S.p.A.
IMPORTANT SAFETY INFORMATION | AiMIFY™ Software
AiMIFY-enhanced images should not be used alone to assist patient diagnosis. The standard post-contrast image must always be reviewed first before using AiMIFY-enhanced images for patient diagnosis.
Please be aware that AiMIFY is not intended for artifact reduction, such as metal artifact and motion artifact. Enhanced images may have similar artifacts as input images.
Reference:
1. Data on file, Subtle Medical, Inc.